Which of the Following Describes a Spontaneous Reaction Apex
The reaction is nonspontaneous at room temperature. 2 See answers Advertisement Advertisement milkywaay0809 milkywaay0809 Answer.

Which Of The Following Describes A Nonspontaneous Reaction A The Gibbs Free Energy Is More Than Brainly Com
Use the following equation.

. What is a chemical reaction that absorbs heat called. Which of the following is the best definition of radioactive decay. A reaction is spontaneous if delta G is negative.
Learn vocabulary terms and more with flashcards games and other study tools. Use the following equation. What is S in the equation G H - TS.
Reason Δ H of the endothermic reaction increases with increase in temperature. In endothermic reactions energy is absorbed by a. Delta G delta H - TdeltaS.
Above 400 K ΔG will become negative and the reaction will become spontaneous. For a reaction to be spontaneous it must have both of these factors. The reaction is spontaneous.
The Gibbs free energy for the reaction is positive. Now we have four possible situations depending on the signs of ΔH and ΔS. A reaction will always be spontaneous under any temperature only if the change in enthalpy delta H is negative and the change in entropy delta S is positive.
In chemical reactions these are called catalysts. The Gibbs free energy is more than the activation energy. The heat released or absorbed in a reaction.
The second is energy. Hf -206 kJmol. It is not spontaneous.
Radioactive decay is the splitting of a nucleus amounts of energy. If ΔG 0 the reaction is not spontaneous in the forward direction but it is spontaneous in the reverse direction. If ΔH is negative and ΔS is positive the reaction is spontaneous at all temperatures because the change in Gibbs free energy is always negative.
A reaction is spontaneous if delta G is negative. Which statement describes a reaction at 298 K if H 31 kJmol S 0093 kJmolK. For the reactionABΔHveΔSveThis reaction is.
If ΔG 0 the reaction is spontaneous in the forward direction. Which of the following is incorrect. The free energy change for the reaction and its spontaneous.
Thus in a chemical equation or reaction products will have more energy than the reactants. The redox reaction in the electrolytic cell is not spontaneous. Which reaction shows that the enthalpy of formation of H2S is.
Which of the following is not correct. Electrical energy has to be supplied to it in order to initiate the reaction. The first of two factors that determine whether a reaction is spontaneous or non-spontaneous is entropy.
What is the term to define all. A an exothermic graph. Delta G delta H - TdeltaS.
The enthalpy change for a certain reaction at 300 K is -150 Kcal mol 1. The change in entropy between products and reactants in a reaction. How do you determine if a reaction is always spontaneous.
Which of the following describes an endothermic reaction. If ΔG 0 the reaction is at equilibrium. Start studying 524 Quiz.
Which of the following describe a spontaneous reaction. The entropy change under these conditions is -72 cal K 1 mol 1. Determine whether the following reaction will be spontaneous or nonspontaneous under standard conditions.
How do you tell if a reaction is Nonspontaneous at all temperatures. Which of the following best describes electroplating. The reaction does not need added energy to occur.
Radioactive decay is the loss of mass that occurs when an atom is formed B. H2 g S s -- H2S 206 kJ. Many endothermic reactions that are not spontaneous at room temperature become spontaneous at high temperatures.
If ΔH is negative and ΔS is positive the reaction is spontaneous at all temperatures because the change in Gibbs free energy is always negative. What is the enthalpy of reaction. It is a matter of where these take place as to which term is used.
The reaction that takes place in the galvanic cell is spontaneous which is responsible for the electrical energy that is produced. It predicts whether or not a reaction will be spontaneous. The Gibbs free energy is less than the activation energy.
Radioactive decay is the release of energy resulting from a chemical reaction. Which of the following describes a nonspontaneous reaction. Contrastingly if ΔH is positive and ΔS is negative the reaction is nonspontaneous at all temperatures as written.
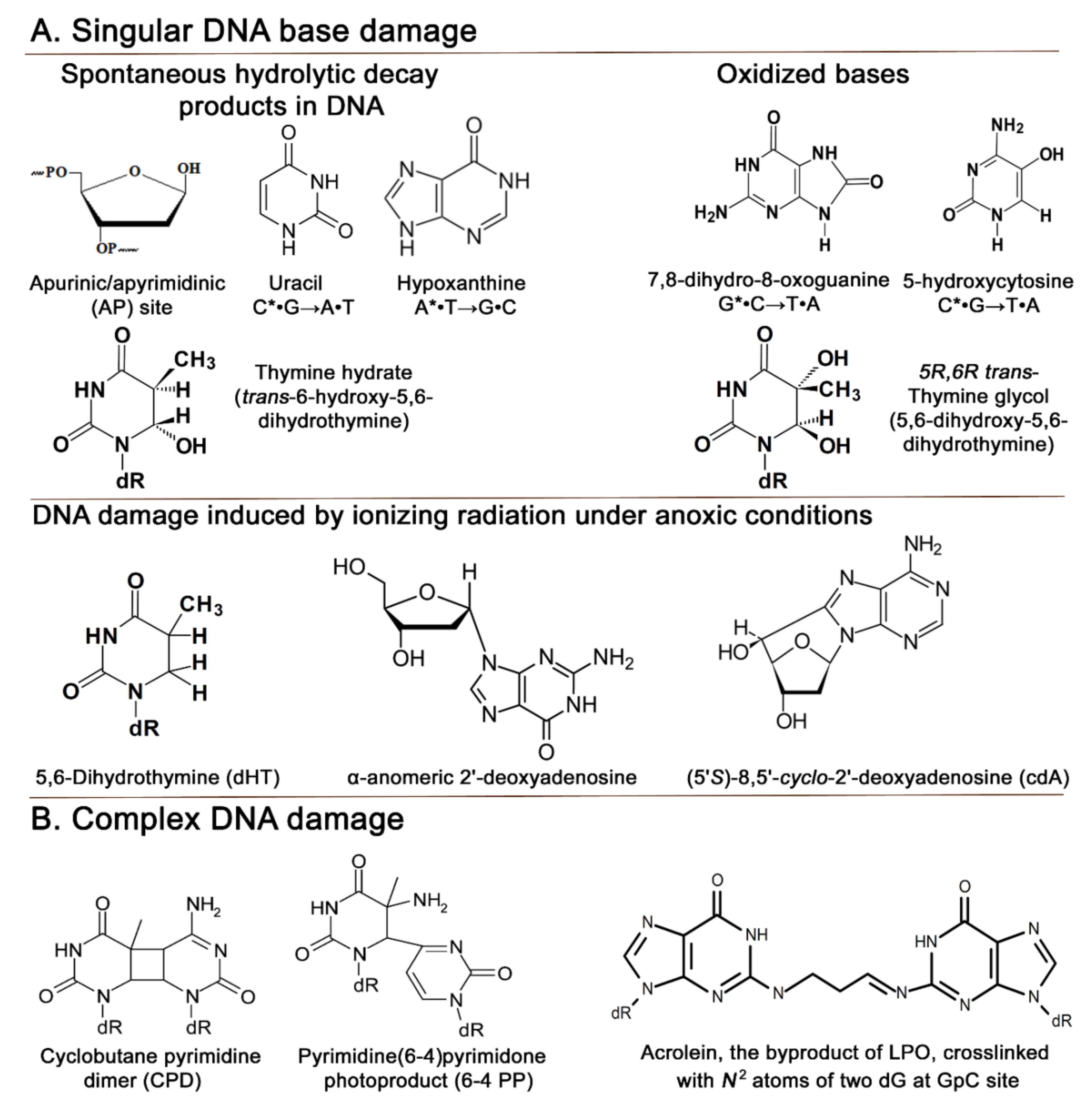
Cells Free Full Text Evolutionary Origins Of Dna Repair Pathways Role Of Oxygen Catastrophe In The Emergence Of Dna Glycosylases Html

Which Of The Following Describes A Spontaneous Reaction Brainly Com

Spontaneous Reactions And Free Energy Ck 12 Foundation
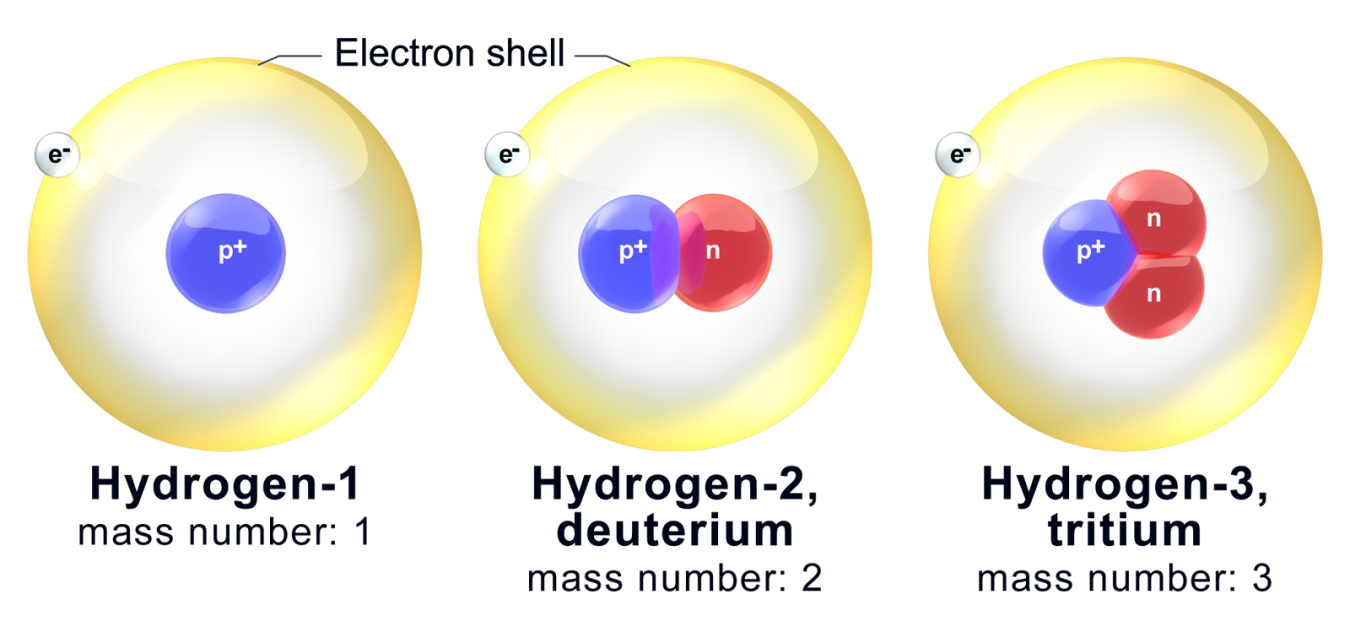
Doe Explains Isotopes Department Of Energy

Chaperonin Assisted Protein Folding A Chronologue Quarterly Reviews Of Biophysics Cambridge Core

Life Dna Rna And Protein Britannica
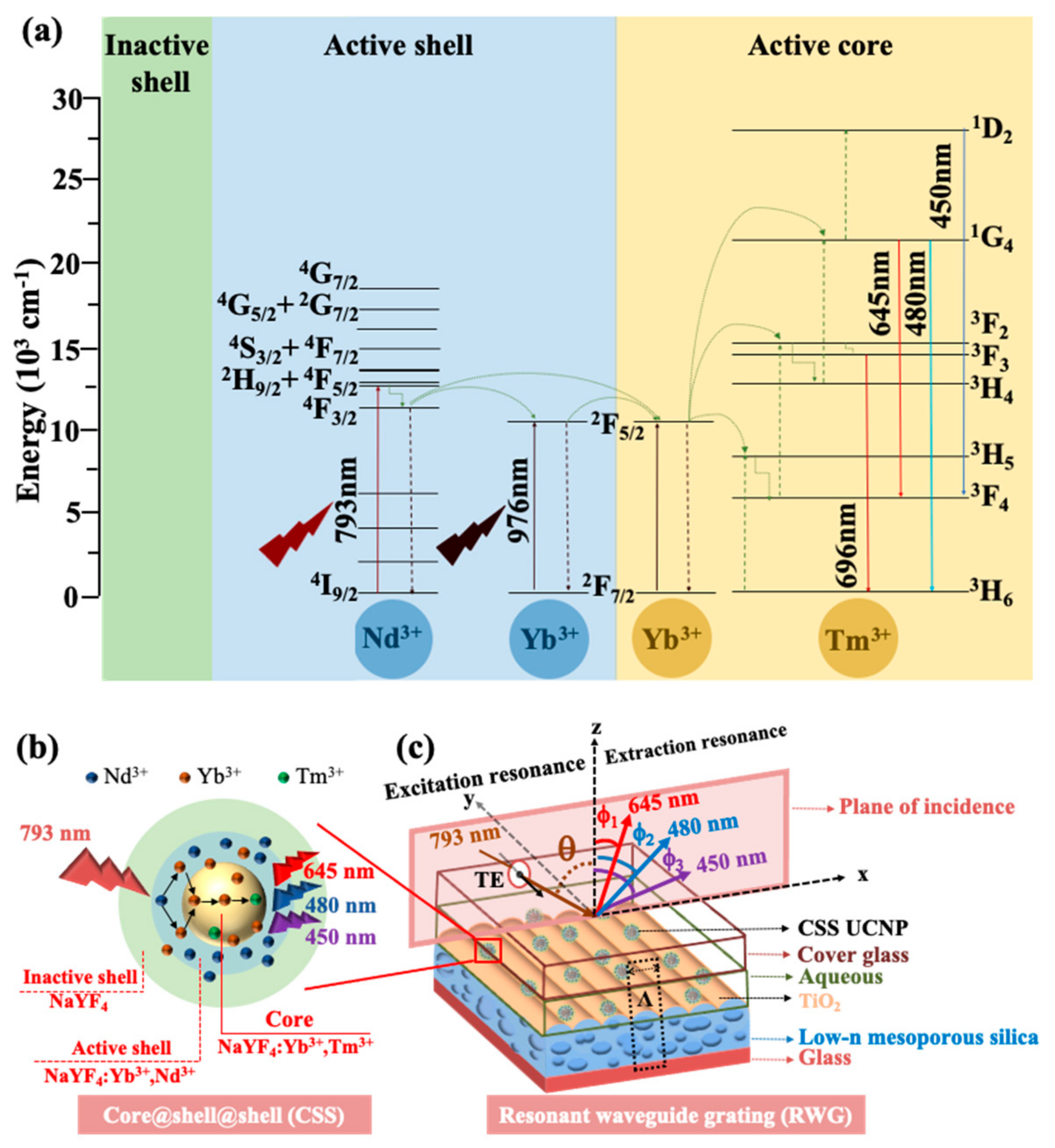
Crystals Free Full Text A Synergy Approach To Enhance Upconversion Luminescence Emission Of Rare Earth Nanophosphors With Million Fold Enhancement Factor Html

Diagnostics Free Full Text Azygos Lobe Prevalence Of An Anatomical Variant And Its Recognition Among Postgraduate Physicians Html

Which Of The Following Describes A Spontaneous Reaction Brainly Com
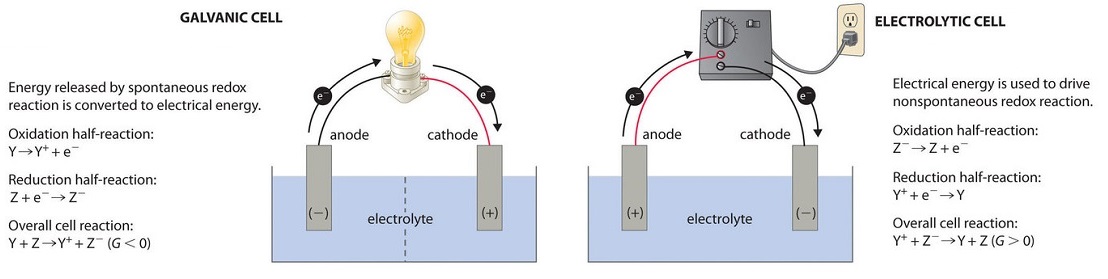
19 3 Voltaic Or Galvanic Cells Generating Electricity From Spontaneous Chemical Reactions Chemistry Libretexts

Jcm Free Full Text Reference Ranges Of Left Ventricular Hemodynamic Forces In Healthy Adults A Speckle Tracking Echocardiographic Study Html

Spontaneous Reactions And Free Energy Ck 12 Foundation

Results From Comparing The Frequencies Of Spontaneous And Posed Facial Download Scientific Diagram
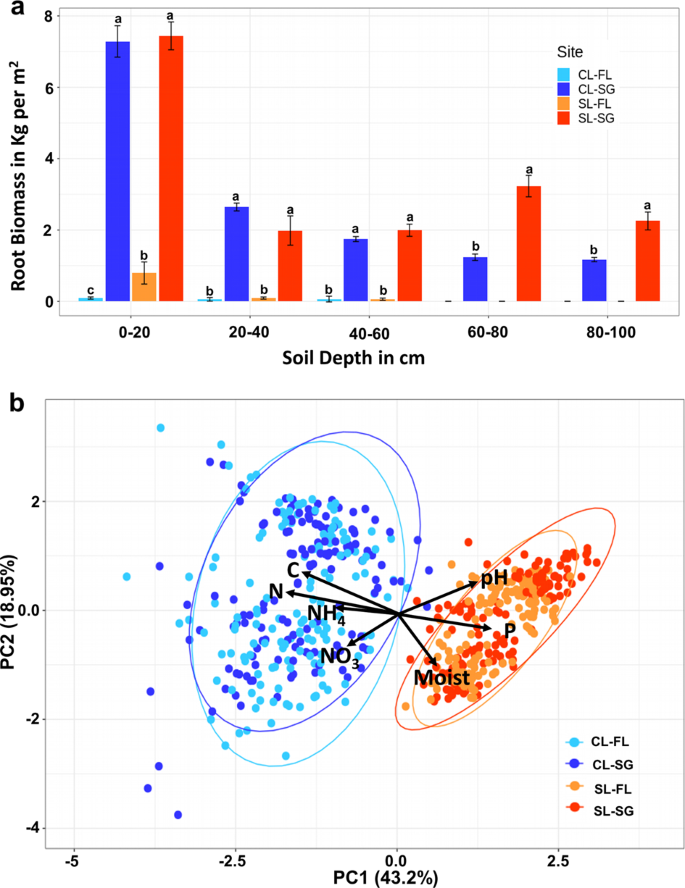
Conversion Of Marginal Land Into Switchgrass Conditionally Accrues Soil Carbon But Reduces Methane Consumption The Isme Journal
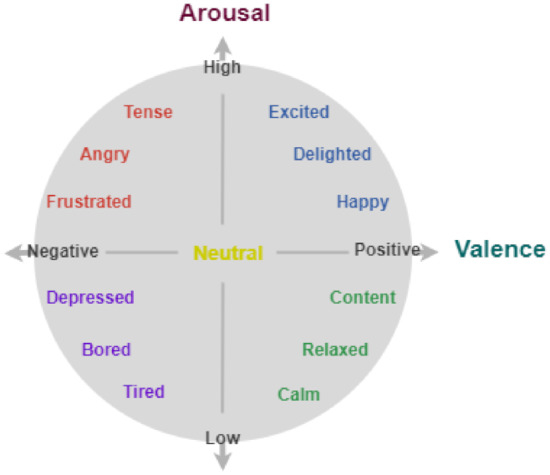
Sensors Free Full Text Macro And Micro Expressions Facial Datasets A Survey Html

2022 Southern Medical Research Conference Journal Of Investigative Medicine

15 8 Le Chatelier S Principle How A System At Equilibrium Responds To Disturbances Chemistry Libretexts
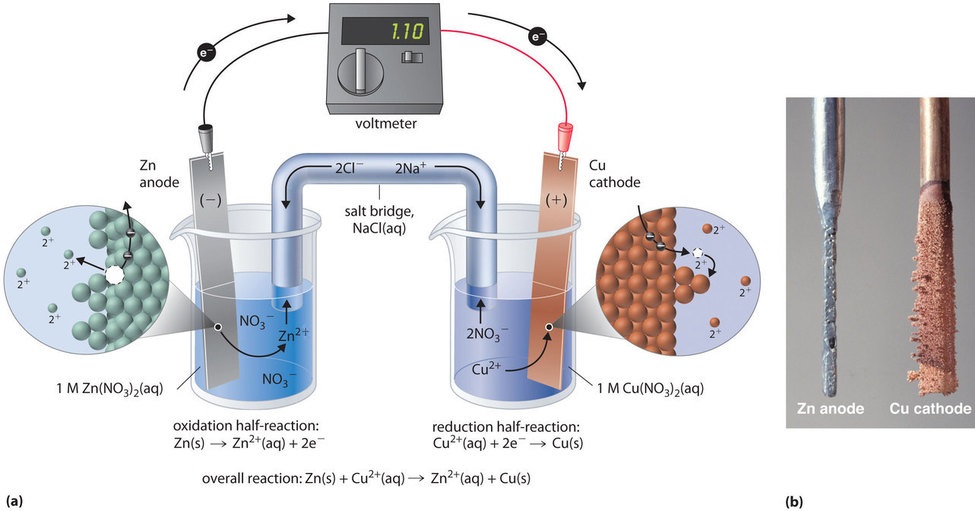
19 3 Voltaic Or Galvanic Cells Generating Electricity From Spontaneous Chemical Reactions Chemistry Libretexts
Comments
Post a Comment